Hubly Surgical
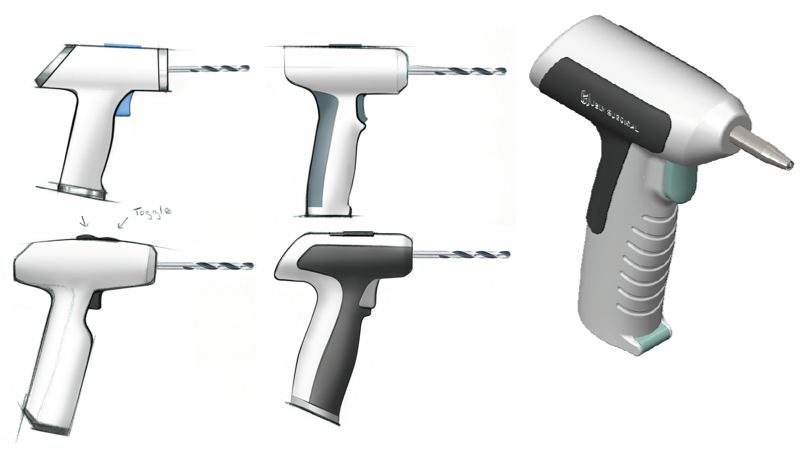
Hubly Surgical’s mission is to improve patient outcomes and increase quality of care for surgical procedures, inclusively, across standard and underserved settings. Hubly is developing an integrated electric drill system designed to streamline bedside intracranial access, decrease operating room reliance, and facilitate treatment for emerging indications.
What we delivered:
510(k) submission, FDA response interaction, and 510(k) clearance (May 2023)
Design and Development Plan
Market / Product Requirements Specifications (Design Inputs)
Risk Management Plan
Preliminary Usability Evaluation
Preliminary Hazard Analysis
Quality Plan
Software Development Plan
Quality Management System Procedures
Electromechanical Product Design
Intellectual Property development
Design Verification Strategy & Execution
FDA Small Business Registration
3rd Party Manufacturer Engagement
Industrial Design
3D Renderings
CAD models
PCB Design gerber files
Packaging and Labeling Design
Product Photography
Usability Study Design & Execution
Working with the team at Ontogen Medtech has been amazing. Each team member I worked with and spoke to is clear, communicative, and flexible. They framed out our Quality System that fits exactly what we need right now, led the charge on further development of our flagship product, and are carving out additional intellectual property for us. Would 100% recommend. We definitely plan to use Ontogen in the future.
- Casey Grage, Hubly Surgical (Entrepreneur, Founder & CEO)